-
Fil d’actualités
- EXPLORER
-
Pages
-
Groupes
-
Evènements
-
Blogs
-
Offres
-
Emplois
-
Courses
North America Medical Device Testing Market Trends, Challenges, Key Suppliers Analysis and Growth By 2025 - 2032
 Executive Summary North America Medical Device Testing Market :
Executive Summary North America Medical Device Testing Market :
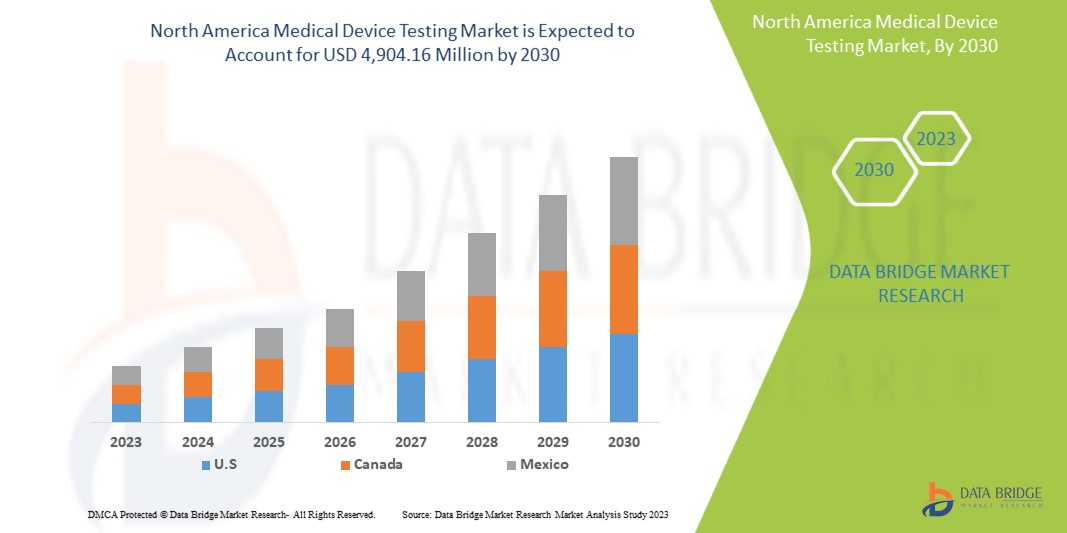
Data Bridge Market Research analyzes that the North America medical device testing market is expected to reach the value of USD 4,904.16 million by 2030, at a CAGR of 12.0% during the forecast period.
This North America Medical Device Testing Market report aids to establish correlative relationship between the product brand and consumers’ needs and preferences. This market research report is a comprehensive analysis on the study of industry. Market research covered in this report helps the management of a firm in planning by providing accurate and up- to-date information about the consumer’s demands, their changing tastes, attitudes, preferences, and buying intentions etc. Further, manufacturer can adjust production according to the conditions of demand which are analysed here. It also supports to secure economies in the distribution of products and find out the best way of approaching the potential. With the data covered in this North America Medical Device Testing Market report, marketing of goods can be made efficient and economical which leads to elimination of all type of wastage.
This North America Medical Device Testing Market report makes focus on the more important aspects of the market like what the market recent trends are. The market study provides details of drivers and restraints for the North America Medical Device Testing Market with the help of SWOT analysis, along with the impact they have on the demand over the forecast period. It provides guidelines about planning of advertising and sales promotion efforts. Furthermore, the North America Medical Device Testing Market report helps the manufacturer in finding out the effectiveness of the existing channels of distribution, advertising programmes or media, selling methods and the best way of distributing the goods to the eventual consumers.
Discover the latest trends, growth opportunities, and strategic insights in our comprehensive North America Medical Device Testing Market report. Download Full Report: https://www.databridgemarketresearch.com/reports/north-america-medical-device-testing-market
North America Medical Device Testing Market Overview
**Segments**
- Based on the service type, the North America medical device testing market can be segmented into testing, inspection, and certification.
- On the basis of technology, the market can be categorized into active implant medical device, active medical device, and non-active medical device.
- By sourcing type, the market can be divided into in-house and outsourced medical device testing.
The North America medical device testing market is expected to witness significant growth in the coming years due to factors such as the increasing demand for medical devices, stringent regulatory requirements, and advancements in healthcare technology. The testing segment is anticipated to dominate the market as manufacturers focus on ensuring the safety and efficacy of their products. The inspection segment is also expected to grow steadily as regulatory bodies emphasize the need for thorough quality control measures. Certification services will witness a surge in demand as companies aim to comply with international standards and gain market access.
**Market Players**
- SGS SA
- Bureau Veritas
- Intertek Group plc
- TÜV SÜD
- Dekra
- Eurofins Scientific
- Avomeen
- Envigo
- Pace Analytical Services, LLC
- Toxikon
- Charles River
- North American Science Associates Inc. (NASA)
- Nelson Laboratories, LLC
- Avomeen Analytical Services
These market players play a crucial role in the North America medical device testing market by offering a wide range of services such as testing, inspection, and certification. SGS SA, Bureau Veritas, and Intertek Group plc are among the leading companies providing comprehensive testing solutions to ensure compliance with regulatory standards. TÜV SÜD and Dekra are also prominent players known for their expertise in medical device testing and certification services. The market is highly competitive, with companies investing in research and development to introduce innovative testing techniques and technologies.
The North America medical device testing market is poised for robust growth driven by several key factors. One significant driver is the increasing demand for medical devices in the region due to a growing aging population and the prevalence of chronic diseases. This demographic trend is fueling the need for continuous innovation and development of new medical devices, thereby creating a substantial market opportunity for testing services. Additionally, stringent regulatory requirements imposed by agencies such as the FDA in the US and Health Canada in Canada are compelling medical device manufacturers to ensure the safety, efficacy, and quality of their products through comprehensive testing procedures. This regulatory compliance aspect is a major factor contributing to the growth of the medical device testing market in North America.
Advancements in healthcare technology are also playing a pivotal role in shaping the market landscape. The rapid evolution of medical devices, including active implantable devices, active medical devices, and non-active medical devices, necessitates thorough testing to validate their performance and safety features. As medical devices become more sophisticated and interconnected, the complexity of testing requirements increases, driving the demand for specialized testing services offered by market players. Moreover, the preference for outsourcing medical device testing services is gaining traction among manufacturers to leverage the expertise and capabilities of third-party testing providers, leading to the segmentation of the market based on sourcing type into in-house and outsourced testing.
In terms of market players, the North America medical device testing market is characterized by intense competition among established companies offering a diverse range of testing, inspection, and certification services. SGS SA, Bureau Veritas, Intertek Group plc, TÜV SÜD, and Dekra are prominent players known for their comprehensive testing solutions and regulatory expertise. These market leaders are continuously investing in research and development initiatives to enhance their service offerings and stay ahead of the evolving regulatory landscape. Collaboration with healthcare organizations, research institutions, and regulatory bodies is also crucial for market players to stay updated on industry trends, standards, and best practices in medical device testing.
In conclusion, the North America medical device testing market is set to experience substantial growth driven by factors such as increasing demand for medical devices, stringent regulatory requirements, and technological advancements in healthcare. Market players need to focus on innovation, collaboration, and compliance to capitalize on the significant opportunities presented by this dynamic and evolving market landscape.The North America medical device testing market is currently witnessing a significant surge in demand due to various factors contributing to its growth trajectory. One of the key drivers propelling market expansion is the increasing emphasis on ensuring the safety, efficacy, and quality of medical devices. With the rise in the aging population and the prevalence of chronic diseases, the demand for innovative medical devices is on the rise, creating a substantial market opportunity for testing services. Additionally, stringent regulatory requirements set by organizations like the FDA and Health Canada are compelling manufacturers to adhere to strict testing protocols to meet compliance standards. This regulatory landscape underscores the importance of thorough testing procedures in validating the performance and safety features of medical devices, further boosting the market for testing services.
Furthermore, advancements in healthcare technology are reshaping the landscape of the medical device testing market in North America. The evolving complexity of medical devices, including active implantable devices, active medical devices, and non-active medical devices, necessitates advanced testing methodologies to ensure their functionality and safety. As the healthcare sector continues to innovate and introduce more sophisticated devices, the demand for specialized testing services provided by market players escalates. Moreover, the trend towards outsourcing medical device testing services is gaining momentum as manufacturers look to leverage external expertise and capabilities for comprehensive testing solutions. This shift in sourcing type, from in-house to outsourced testing, serves to further segment the market and create opportunities for service providers to offer tailored solutions to meet specific testing requirements.
The competitive landscape of the North America medical device testing market is marked by intense rivalry among key players offering a wide array of testing, inspection, and certification services. Established companies like SGS SA, Bureau Veritas, Intertek Group plc, TÜV SÜD, and Dekra are at the forefront of delivering regulatory-compliant testing solutions and leveraging their expertise to meet the evolving demands of the market. These market leaders are heavily investing in research and development endeavors to enhance their service portfolios and stay abreast of regulatory changes. Collaboration with industry stakeholders, research institutions, and regulatory bodies is a pivotal strategy for market players to stay informed about industry trends, standards, and best practices in medical device testing.
In conclusion, the North America medical device testing market presents ample growth prospects fueled by the increasing demand for medical devices, stringent regulatory requirements, and advancements in healthcare technology. Market players need to prioritize innovation, collaboration, and regulatory compliance to capitalize on the burgeoning opportunities in this dynamic and evolving market landscape. By staying attuned to industry trends and customer needs, service providers can position themselves as key players in the competitive marketplace and drive continued growth in the medical device testing sector.
The North America Medical Device Testing Market is highly fragmented, featuring intense competition among both global and regional players striving for market share. To explore how global trends are shaping the future of the top 10 companies in the keyword market.
Learn More Now: https://www.databridgemarketresearch.com/reports/north-america-medical-device-testing-market/companies
DBMR Nucleus: Powering Insights, Strategy & Growth
DBMR Nucleus is a dynamic, AI-powered business intelligence platform designed to revolutionize the way organizations access and interpret market data. Developed by Data Bridge Market Research, Nucleus integrates cutting-edge analytics with intuitive dashboards to deliver real-time insights across industries. From tracking market trends and competitive landscapes to uncovering growth opportunities, the platform enables strategic decision-making backed by data-driven evidence. Whether you're a startup or an enterprise, DBMR Nucleus equips you with the tools to stay ahead of the curve and fuel long-term success.
Key Benefits of the Report:
- This study presents the analytical depiction of the global North America Medical Device Testing Marketindustry along with the current trends and future estimations to determine the imminent investment pockets.
- The report presents information related to key drivers, restraints, and opportunities along with detailed analysis of the global North America Medical Device Testing Market share.
- The current market is quantitatively analyzed from to highlight the Global North America Medical Device Testing Market growth scenario.
- Porter's five forces analysis illustrates the potency of buyers & suppliers in the market.
The report provides a detailed global North America Medical Device Testing Market analysis based on competitive intensity and how the competition will take shape in coming years
Browse More Reports:
Middle East and Africa Breast Reconstruction Market
Global Data Governance Market
Global Electronic Filters Market
North America Fitness Equipment Market
Global Company Secretarial Software Market
Europe Specialty Paper Market
Asia-Pacific Usage Based Insurance Market
Global Air Humidifier Market
Global Automotive Collision Repair Market
Global Epigenetics-Based Kits Market
Global Turmeric Spices Market
Global Vegetable Shortening Market
Europe Persistent Corneal Epithelial Defects Treatment Market
Global Beverage Can Ends Market
Global Book Services Market
Global Hyperacusis Drug Market
Global Recycled Lead Market
Global Neuroblastoma Drugs Market
Global Camouflage Coatings Market
Global Surface Protection Tapes Market
Global Drug Discovery Services Market
Global Intracranial Pressure Monitors Market
Global Unsweetened Almond Milk Market
Global Vasoconstrictor Drugs Market
Global Solar Photovoltaic Glass Market
Middle East and Africa Operating Room Equipment Supplies Market
North America Balsamic Vinegar Market
Europe Laryngoscopes Market
Global Automotive Intelligence Park Assist System Market
Global Anti-Fog Polycarbonate Films and Sheets Market
Global Anti-Counterfeit Packaging Market
Global Recombinant Human Growth Hormone (RHGH) Market
Global Compost Turning Machine Market
Global Veterinary Calcium Supplement Market
Germany Fitness Equipment Market
About Data Bridge Market Research:
An absolute way to forecast what the future holds is to comprehend the trend today!
Data Bridge Market Research set forth itself as an unconventional and neoteric market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process. Data Bridge is an aftermath of sheer wisdom and experience which was formulated and framed in the year 2015 in Pune.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email:- corporatesales@databridgemarketresearch.com
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Jeux
- Gardening
- Health
- Domicile
- Literature
- Music
- Networking
- Autre
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness
