Asia-Pacific Drug Safety Solutions and Pharmacovigilance Market Challenges: Growth, Share, Value, Size, and Scope By 2032
Executive Summary Asia-Pacific Drug Safety Solutions and Pharmacovigilance Market :
CAGR Value: Data Bridge Market Research analyses that the Asia-Pacific drug safety solutions and pharmacovigilance market which was USD 1,220.34 million in 2022, would rocket up to USD 2,615.51 million by 2030, and is expected to undergo a CAGR of 8.1% during the forecast period.
Asia-Pacific Drug Safety Solutions and Pharmacovigilance Market research report studies various parameters throughout the report which analyses the market status in detail. It offers key measurements, status of the manufacturers and is a major source of direction for the businesses and organizations. Such market insights can be accomplished with this comprehensive Asia-Pacific Drug Safety Solutions and Pharmacovigilance Market research report which takes into account all the aspects of current and future market. In addition, Asia-Pacific Drug Safety Solutions and Pharmacovigilance Market research report predicts the size of the market with information on key vendor revenues, development of the industry by upstream & downstream, industry progress, key companies, segment type & market application.
The report carefully studies market definition, market segmentation, competitive analysis and key developments in the market. This Asia-Pacific Drug Safety Solutions and Pharmacovigilance Market research report consists of latest, comprehensive and most up-to-date market information and a precious data. Asia-Pacific Drug Safety Solutions and Pharmacovigilance Market report gives the market insights which help to have a more precise understanding of the market landscape, issues that may impose on the industry in the future, and how to position specific brands in the best way. It also studies the market status, growth rate, future trends, market drivers, opportunities and challenges, risks and entry barriers, sales channels, and distributors with the help of SWOT analysis and Porter's Five Forces Analysis.
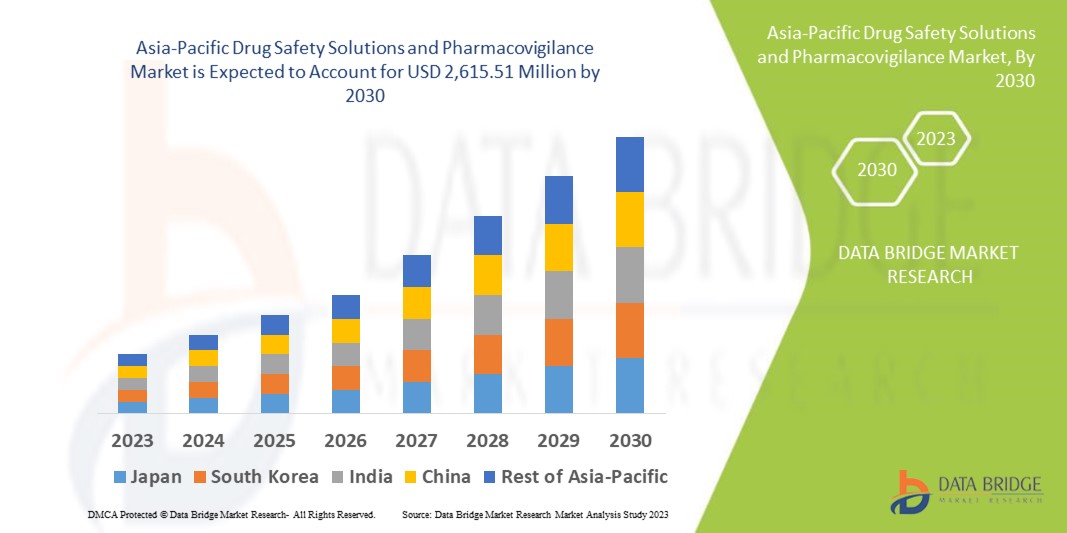
Discover the latest trends, growth opportunities, and strategic insights in our comprehensive Asia-Pacific Drug Safety Solutions and Pharmacovigilance Market report. Download Full Report: https://www.databridgemarketresearch.com/reports/asia-pacific-drug-safety-solutions-and-pharmacovigilance-market
Asia-Pacific Drug Safety Solutions and Pharmacovigilance Market Overview
**Segments**
- On the basis of type, the Asia-Pacific drug safety solutions and pharmacovigilance market can be segmented into adverse event reporting software, drug safety auditing software, issue tracking software, fully integrated software, and others. The adverse event reporting software segment is expected to witness significant growth due to the increasing focus on patient safety and the need for efficient adverse event management systems. This software helps in the systematic collection, documentation, and analysis of adverse events associated with drugs or medical devices.
- By deployment mode, the market can be divided into on-premise and cloud-based solutions. The cloud-based solutions segment is anticipated to register a higher growth rate during the forecast period. Cloud-based solutions offer scalability, flexibility, and cost-effectiveness, making them increasingly popular among pharmaceutical companies and healthcare organizations in the Asia-Pacific region.
- Based on end-user, the market can be categorized into pharmaceutical and biotechnology companies, contract research organizations (CROs), business process outsourcing (BPO) firms, and healthcare providers. The pharmaceutical and biotechnology companies segment is expected to dominate the market owing to the stringent regulatory requirements for pharmacovigilance and the increasing focus on enhancing drug safety and efficacy.
**Market Players**
- Some of the key players operating in the Asia-Pacific drug safety solutions and pharmacovigilance market include Oracle Corporation, IQVIA, Cognizant, ArisGlobal, DXC Technology Company, TAKE Solutions Limited, Sparta Systems, Inc., Sarjen Systems Pvt. Ltd., Linical Accelovance, and EXTEDO. These companies are focusing on expanding their product portfolios, enhancing technological capabilities, and collaborating with other players to gain a competitive edge in the market.
The Asia-Pacific drug safety solutions and pharmacovigilance market is witnessing significant growth due to factors such as increasing regulatory requirements for drug safety monitoring, rising incidences of adverse drug reactions, and the growing adoption of advanced pharmacovigilance software solutions. The region is home to a large number of pharmaceutical and biotechnology companies, driving the demand for drug safety solutions and pharmacovigilance services. The increasing investments in healthcare infrastructure and the rising focus on patient safety are also contributing to the market growth in the Asia-Pacific region.
Furthermore, advancements in technology such as artificial intelligence (AI), machine learning, and big data analytics are revolutionizing the drug safety and pharmacovigilance landscape in the Asia-Pacific region. These technologies are enabling companies to improve the efficiency and accuracy of adverse event reporting, trend analysis, signal detection, and risk management. Additionally, the shift towards proactive pharmacovigilance approaches, real-time monitoring of drug safety data, and the integration of mobile health technologies are driving the demand for innovative drug safety solutions in the region.
In conclusion, the Asia-Pacific drug safety solutions and pharmacovigilance market is poised for robust growth in the coming years, fueled by regulatory mandates, technological advancements, and the increasing focus on patient safety. Market players are striving to capitalize on these opportunities by offering comprehensive solutions that cater to the evolving needs of pharmaceutical companies, CROs, and healthcare providers in the region.
The Asia-Pacific drug safety solutions and pharmacovigilance market is experiencing a significant transformation driven by various factors that are shaping the landscape of pharmacovigilance services in the region. One notable trend is the increasing emphasis on real-world data and evidence-based medicine, which is leading to a shift towards more proactive and data-driven pharmacovigilance approaches. Pharmaceutical companies and healthcare organizations are increasingly leveraging advanced analytics tools and technologies to extract valuable insights from vast amounts of data generated from clinical trials, medical records, and patient reports.
Moreover, the rising prevalence of chronic diseases and the growing demand for personalized medicine are driving the need for robust drug safety solutions that can effectively monitor and manage the safety profiles of medications. This trend is influencing market players to innovate and develop solutions that not only ensure regulatory compliance but also enable proactive risk assessment and mitigation strategies. The integration of real-time monitoring capabilities, AI-powered signal detection algorithms, and predictive modeling techniques are becoming essential features of modern pharmacovigilance software platforms in the Asia-Pacific region.
Another important aspect shaping the market is the increasing focus on patient-centric pharmacovigilance practices. Patient safety and engagement have become paramount considerations for pharmaceutical companies and regulatory authorities, leading to the implementation of patient-centric reporting systems and initiatives. This shift towards patient-centered pharmacovigilance is driving the demand for solutions that empower patients to report adverse events directly, participate in risk management programs, and access accurate information on medication safety.
Furthermore, the regulatory landscape and compliance requirements in the Asia-Pacific region are evolving rapidly, necessitating companies to invest in comprehensive pharmacovigilance solutions that can adapt to changing regulations and guidelines. Market players are focusing on enhancing the interoperability of their software platforms, ensuring seamless data exchange, and compliance with regional reporting standards. Collaboration with regulatory bodies, industry associations, and technology partners is becoming crucial for staying abreast of regulatory developments and ensuring compliance in the dynamic market environment.
Overall, the Asia-Pacific drug safety solutions and pharmacovigilance market is poised for continued growth and innovation driven by the convergence of technology advancements, regulatory changes, and shifting healthcare dynamics. Market players that can anticipate and respond to these trends effectively will be well-positioned to capitalize on the emerging opportunities in the region's pharmacovigilance landscape. Continuous investment in research and development, strategic partnerships, and customer-centric solutions will be key strategies for sustaining competitiveness and achieving long-term growth in the dynamic Asia-Pacific market.The Asia-Pacific drug safety solutions and pharmacovigilance market is currently experiencing a paradigm shift driven by several key factors that are reshaping the landscape of pharmacovigilance services in the region. One prominent trend is the rising importance placed on real-world data and evidence-based medicine, leading to a transition towards more proactive and data-driven pharmacovigilance approaches. Pharmaceutical companies and healthcare organizations are increasingly leveraging advanced analytics tools and technologies to extract valuable insights from a plethora of data derived from clinical trials, medical records, and patient reports.
Additionally, the increasing prevalence of chronic diseases and the escalating demand for personalized medicine are propelling the necessity for robust drug safety solutions capable of effectively monitoring and managing the safety profiles of medications. This trend is compelling market players to innovate and develop solutions that not only guarantee regulatory compliance but also facilitate proactive risk assessment and mitigation strategies. The integration of real-time monitoring capabilities, AI-powered signal detection algorithms, and predictive modeling techniques are evolving into indispensable features of modern pharmacovigilance software platforms in the Asia-Pacific region.
Another pivotal aspect influencing the market is the growing focus on patient-centric pharmacovigilance practices. Patient safety and engagement have emerged as critical considerations for pharmaceutical companies and regulatory authorities, culminating in the implementation of patient-centric reporting systems and initiatives. This shift towards patient-centered pharmacovigilance is fueling the demand for solutions that empower patients to directly report adverse events, partake in risk management programs, and access accurate information on medication safety.
Moreover, the evolving regulatory landscape and compliance requirements in the Asia-Pacific region are compelling companies to invest in comprehensive pharmacovigilance solutions that can adapt to changing regulations and guidelines. Market players are prioritizing the enhancement of the interoperability of their software platforms to ensure seamless data exchange and compliance with regional reporting standards. Collaboration with regulatory bodies, industry associations, and technology partners is becoming imperative to stay abreast of regulatory developments and ensure compliance in the dynamic market environment.
Overall, the Asia-Pacific drug safety solutions and pharmacovigilance market are poised for continued growth and innovation propelled by the convergence of technological advancements, regulatory shifts, and evolving healthcare dynamics. Companies that can anticipate and effectively respond to these trends will be well-positioned to capitalize on the emerging opportunities in the region's pharmacovigilance landscape. Continuous investment in research and development, strategic partnerships, and customer-centric solutions will be crucial strategies for maintaining competitiveness and achieving sustainable growth in the dynamic Asia-Pacific market.
The Asia-Pacific Drug Safety Solutions and Pharmacovigilance Market is highly fragmented, featuring intense competition among both global and regional players striving for market share. To explore how global trends are shaping the future of the top 10 companies in the keyword market.
Learn More Now: https://www.databridgemarketresearch.com/reports/asia-pacific-drug-safety-solutions-and-pharmacovigilance-market/companies
DBMR Nucleus: Powering Insights, Strategy & Growth
DBMR Nucleus is a dynamic, AI-powered business intelligence platform designed to revolutionize the way organizations access and interpret market data. Developed by Data Bridge Market Research, Nucleus integrates cutting-edge analytics with intuitive dashboards to deliver real-time insights across industries. From tracking market trends and competitive landscapes to uncovering growth opportunities, the platform enables strategic decision-making backed by data-driven evidence. Whether you're a startup or an enterprise, DBMR Nucleus equips you with the tools to stay ahead of the curve and fuel long-term success.
Influence of the Asia-Pacific Drug Safety Solutions and Pharmacovigilance Market Report:
- Comprehensive assessment of all opportunities and risk in the Asia-Pacific Drug Safety Solutions and Pharmacovigilance Market
- Lead Asia-Pacific Drug Safety Solutions and Pharmacovigilance Market recent innovations and major events
- Detailed study of business strategies for growth of the Asia-Pacific Drug Safety Solutions and Pharmacovigilance Market market-leading players
- Conclusive study about the growth plot of Asia-Pacific Drug Safety Solutions and Pharmacovigilance Market for forthcoming years
- In-depth understanding of Asia-Pacific Drug Safety Solutions and Pharmacovigilance Market -particular drivers, constraints and major micro markets
- Favourable impression inside vital technological and Asia-Pacific Drug Safety Solutions and Pharmacovigilance Marketlatest trends striking the Cannabis Seeds Market
Browse More Reports:
Global Railway Wiring Harness Market
Global Main Landing Gears Market
Global Cat Litter Market
Global Rack Mounted Video Encoders Market
Global Patient Derived Xenografts Market
Australia Cancer Treatment Market
Global Cloud Computing Insuretech Market
Global Mid-and High-Level Precision Global Positioning System (GPS) Receiver Market
Global Beri Beri Treatment Market
Global Luxury Cosmetics Market
North America Antiblock Additive Market
Global Preeclampsia Laboratory Testing Market
Global Cocoa Products Market
Middle East and Africa Food Grade and Animal Feed Grade Salt Market
Global Plant-Based Oils Market
Global Airport Access Control Market
Middle East and Africa Cocoa Products Market
Global Biopharmaceuticals Manufacturing Consumables Testing Market
Global Secondary Macronutrients Market
Global Fruit Flavor Granola Bars Market
Global Advanced Structural Ceramics Market
Europe Flat Glass Market
Global Broadband Data Card Market
Global Ceramic Fiber Market
Global Smart Utilities Market
Global Milk Minerals Market
Global Artificial Pancreas Device Systems Market
Global Surgical Navigation Systems Software Market
Global Stain Removers Market
Asia-Pacific Agricultural Lubricants Market
Global Micronized Salt Market
Asia-Pacific Drug Safety Solutions and Pharmacovigilance Market
About Data Bridge Market Research:
An absolute way to forecast what the future holds is to comprehend the trend today!
Data Bridge Market Research set forth itself as an unconventional and neoteric market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process. Data Bridge is an aftermath of sheer wisdom and experience which was formulated and framed in the year 2015 in Pune.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email:- corporatesales@databridgemarketresearch.com
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Παιχνίδια
- Gardening
- Health
- Κεντρική Σελίδα
- Literature
- Music
- Networking
- άλλο
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness
