Pediatric Clinical Trials Market to Hit USD 30.32 Billion by 2032
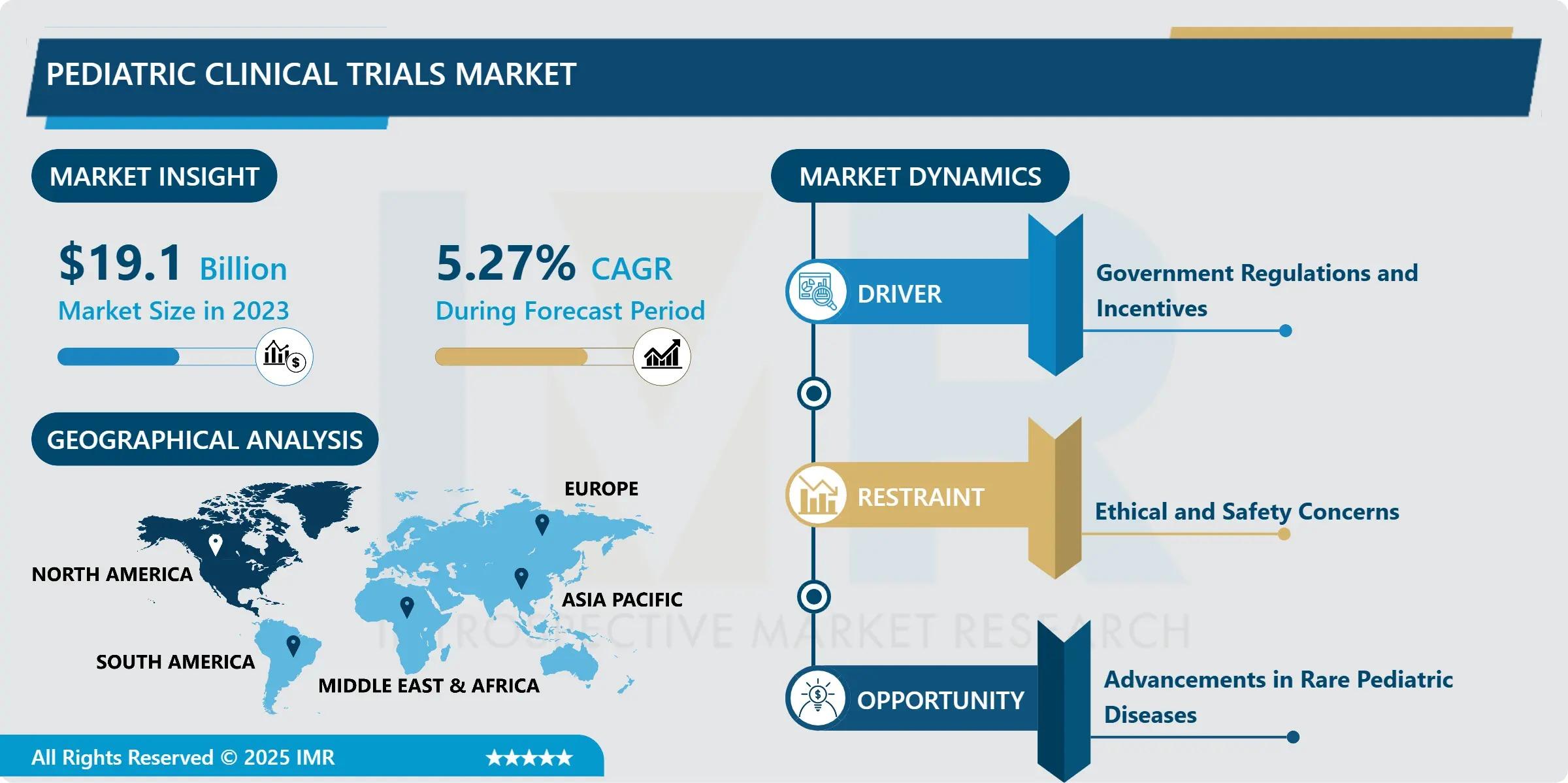
“According to a new report published by Introspective Market Research, Pediatric Clinical Trials Market by Phase, Study Design, Indication, and End User, The Global Pediatric Clinical Trials Market Size Was Valued at USD 19.10 Billion in 2023 and is Projected to Reach USD 30.32 Billion by 2032, Growing at a CAGR of 5.27% from 2024–2032.”
The Pediatric Clinical Trials Market plays a critical role in advancing child-specific therapies, vaccines, and medical devices. Unlike adult clinical trials, pediatric trials require specialized protocols, ethical considerations, and age-appropriate formulations to ensure safety and efficacy across neonates, infants, children, and adolescents. These trials are essential for addressing unmet pediatric healthcare needs and reducing off-label drug use.
Rising prevalence of pediatric diseases, including rare genetic disorders, oncology indications, and chronic conditions such as asthma and diabetes, has significantly increased demand for pediatric-focused clinical research. Regulatory agencies worldwide are encouraging pediatric studies through mandates and incentive programs, supporting pharmaceutical and biotechnology companies in conducting age-appropriate trials.
Additionally, technological advancements in trial design, decentralized clinical trials, and improved patient recruitment strategies are enhancing trial efficiency. The growing involvement of contract research organizations (CROs) and academic research institutes further strengthens the pediatric clinical trials ecosystem, driving steady market growth globally.
Market Segmentation
The Pediatric Clinical Trials Market is segmented into Phase, Study Design, and End User.
By Phase, the market is categorized into Phase I, Phase II, Phase III, and Phase IV.
By Study Design, the market is categorized into Interventional Studies, Observational Studies, and Expanded Access Studies.
By End User, the market is categorized into Pharmaceutical & Biotechnology Companies, Contract Research Organizations (CROs), and Academic Research Institutes.
Growth Driver
One of the key growth drivers of the Pediatric Clinical Trials Market is the increasing regulatory emphasis on pediatric drug development. Regulatory bodies such as the FDA and EMA have implemented pediatric investigation plans and exclusivity incentives, compelling pharmaceutical companies to conduct pediatric trials. These initiatives aim to ensure safe and effective therapies for children while reducing reliance on off-label prescriptions. As a result, sponsors are allocating higher budgets toward pediatric research programs, accelerating trial activity and supporting consistent market expansion.
Market Opportunity
A major market opportunity lies in the growing adoption of decentralized and hybrid pediatric clinical trial models. Virtual monitoring tools, wearable devices, and remote data collection reduce patient burden and improve enrollment and retention rates among pediatric populations. These innovations are particularly beneficial for rare disease trials and geographically dispersed participants. As digital health technologies mature, sponsors and CROs can conduct more efficient, patient-centric pediatric trials, unlocking new growth avenues across emerging and developed regions.
Detailed Segmentation
Pediatric Clinical Trials Market, Segmentation
The Pediatric Clinical Trials Market is segmented on the basis of Phase, Study Design, and End User.
Phase
The Phase segment is further classified into Phase I, Phase II, and Phase III. Among these, the Phase III sub-segment accounted for the highest market share in 2023. Phase III pediatric trials involve large patient populations to confirm treatment efficacy and monitor adverse effects before regulatory approval. The growing pipeline of pediatric drugs and vaccines, particularly in oncology and infectious diseases, has increased demand for late-stage trials, contributing to the dominance of this segment.
Study Design
The Study Design segment is further classified into Interventional Studies, Observational Studies, and Expanded Access Studies. Among these, the Interventional Studies sub-segment accounted for the highest market share in 2023. Interventional studies are crucial for evaluating the safety and effectiveness of new pediatric therapies. Their structured protocols, regulatory acceptance, and direct therapeutic outcomes make them the preferred choice for sponsors, driving strong growth in this segment.
Some of The Leading/Active Market Players Are-
• IQVIA (USA)
• ICON plc (Ireland)
• PPD, Inc. (USA)
• Parexel International Corporation (USA)
• Syneos Health (USA)
• Charles River Laboratories (USA)
• Medpace Holdings, Inc. (USA)
• PRA Health Sciences (USA)
• WuXi AppTec (China)
• Covance Inc. (USA)
• Worldwide Clinical Trials (USA)
• Pharmaceutical Product Development, LLC (USA)
• Clinipace Inc. (USA)
• Novotech Health Holdings (Australia)
• Frontage Laboratories (USA)
and other active players.
Key Industry Developments
In March 2024, a leading CRO expanded its pediatric clinical trial services to support rare disease research.
The expansion focused on enhancing pediatric patient recruitment, decentralized trial capabilities, and regulatory consulting services, aiming to accelerate drug development timelines for rare pediatric conditions.
In September 2023, a global pharmaceutical company initiated multiple pediatric oncology trials across North America and Europe.
These trials targeted novel immunotherapies and precision medicines, highlighting growing investment in pediatric cancer research and advanced therapeutic approaches.
Key Findings of the Study
• Phase III trials dominate the market due to high late-stage drug pipelines
• North America leads owing to strong regulatory support and research funding
• Regulatory mandates are a major growth driver
• Decentralized trial models are emerging as a key trend
More Info:- https://introspectivemarketresearch.com/reports/pediatric-clinical-trials-market/
About Us
At Introspective Market Research Private Limited, we are a forward-thinking research consulting firm committed to driving growth in the Pediatric Clinical Trials Market. With deep insights, strategic solutions, and holistic research, we empower businesses to achieve success and dominance in the global Pediatric Clinical Trials industry.
📞 Contact Us
Introspective Market Research Pvt. Ltd.
📞 Phone: +91-91753-37569
📧 Email: sales@introspectivemarketresearch.com
🌐 Web: www.introspectivemarketresearch.com
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Games
- Gardening
- Health
- Home
- Literature
- Music
- Networking
- Other
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness
