EConsent in Healthcare Market Strategic Analysis of Cloud and On-Premise Solutions 2030
EConsent in Healthcare Market: Digital Transformation of Patient Consent and Clinical Research
The EConsent in Healthcare Market represents a fundamental shift in how informed consent is delivered, documented, and managed across clinical trials, medical procedures, and digital healthcare services. By replacing traditional paper-based consent forms with interactive electronic platforms, eConsent solutions are reshaping patient engagement, regulatory compliance, and operational efficiency across the global healthcare ecosystem.
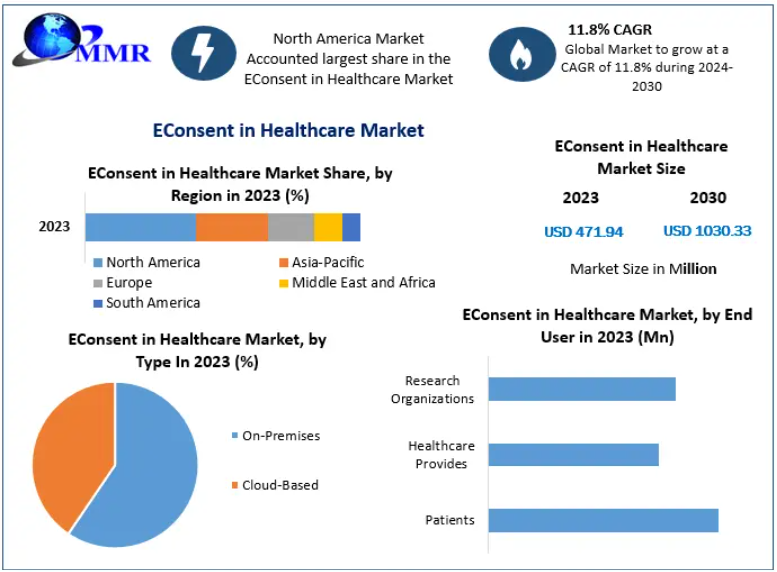
Valued at USD 471.94 million in 2023, the market is projected to grow at a CAGR of 11.8% from 2024 to 2030, reaching approximately USD 1.03 billion by 2030. This expansion reflects the accelerating digitization of healthcare, rising clinical trial activity, growing telehealth adoption, and increasing regulatory emphasis on data security and patient transparency.
Market Overview: From Paper Forms to Intelligent Digital Consent
EConsent systems enable patients and research participants to review, understand, and provide informed consent through secure electronic platforms. These solutions deliver content through multimedia formats such as videos, graphics, animations, and interactive questionnaires, improving comprehension and accommodating diverse learning preferences.
Unlike traditional paper consent, which is prone to errors, delays, and documentation gaps, eConsent provides automated tracking, real-time validation, secure archiving, and audit-ready records. Patients can access consent materials via smartphones, tablets, or web applications, making the process more accessible, consistent, and patient-centric.
The market is gaining strong traction in clinical trials, pharmaceutical research, hospitals, telemedicine platforms, and health information exchange networks, positioning eConsent as a core component of digital health infrastructure.
To know the most attractive segments, click here for a free sample of the report:https://www.maximizemarketresearch.com/request-sample/219294/
Key Market Drivers: Efficiency, Engagement, and Regulatory Alignment
The primary driver of the eConsent market is the healthcare industry’s growing focus on operational efficiency and patient engagement. Clinical research organizations and hospitals are under pressure to accelerate enrollment, reduce administrative burden, and improve compliance accuracy. EConsent solutions streamline workflows, shorten consent cycles, and minimize reconciliation errors.
Government and regulatory support plays a crucial role in market expansion. Regulations such as HIPAA in the United States and GDPR in Europe emphasize patient privacy, data security, and transparency, creating strong incentives for secure digital consent platforms. Many regulatory agencies now recognize electronic consent as a valid and often preferred method of documentation.
The rapid growth of mobile health technologies further supports adoption. Smartphones, tablets, and cloud-based applications allow patients to review consent materials remotely, making eConsent particularly valuable for decentralized trials, remote monitoring programs, and virtual care models.
Technology Evolution: Multimedia, Mobile, and Integrated Platforms
Technological innovation is redefining the functionality of eConsent platforms. Modern systems integrate multimedia education modules, real-time chat features, interactive quizzes, and electronic signatures to improve comprehension and engagement. Multilingual support expands accessibility across global patient populations.
Integration with eClinical platforms, electronic data capture (EDC), electronic patient-reported outcomes (ePRO), and trial management systems creates seamless digital workflows. These connected ecosystems reduce duplication, enhance traceability, and provide sponsors with real-time visibility into enrollment and compliance status.
Security and compliance remain central to platform design. Leading eConsent solutions incorporate encryption, multi-factor authentication, audit trails, and digital identity verification to ensure data integrity and regulatory readiness.
Market Restraints: Awareness Gaps and Technical Complexity
Despite strong momentum, several challenges limit adoption. Lack of awareness among healthcare professionals and patients remains a significant barrier, particularly in emerging markets. Concerns regarding data privacy, cybersecurity, and system reliability can slow institutional adoption.
Technical integration poses another challenge. Many healthcare organizations operate legacy IT systems that require careful alignment with modern eConsent platforms. Ensuring interoperability without disrupting clinical workflows demands investment, expertise, and change management.
Smaller clinics and research centers may also face budget constraints, limiting their ability to deploy advanced digital consent infrastructure.
Emerging Opportunities: Telehealth, Remote Trials, and Patient-Centric Care
The rapid growth of telehealth and remote patient monitoring is creating powerful opportunities for eConsent adoption. Virtual care models require secure, remote-friendly consent processes that can operate across devices and geographies. EConsent enables efficient onboarding and continuous consent management for long-term digital health programs.
Decentralized and hybrid clinical trials represent another high-growth segment. These trials depend heavily on electronic consent to recruit participants remotely, manage protocol updates, and ensure regulatory compliance across distributed study populations.
The broader shift toward patient-centric care is also strengthening demand. Patients increasingly expect transparency, clarity, and digital convenience in their healthcare interactions, positioning eConsent as a foundational engagement tool.
Segment Analysis
By Deployment Type
Cloud-based eConsent platforms dominate the market due to scalability, remote accessibility, and lower infrastructure requirements. On-premises solutions remain relevant for institutions with strict internal data governance policies and customized integration needs.
By Application
Clinical trials represent the largest application segment, driven by regulatory requirements and complex documentation needs. Medical procedures and surgeries follow closely, while telehealth and remote monitoring are the fastest-growing segments. Data sharing and health information exchange are emerging applications supporting integrated care delivery.
By End User
Patients remain the primary users, supported by healthcare providers, research organizations, and clinical coordinators. Research institutions and pharmaceutical sponsors represent major institutional buyers due to high trial volumes and compliance complexity.
To know the most attractive segments, click here for a free sample of the report:https://www.maximizemarketresearch.com/request-sample/219294/
Regional Outlook
North America
North America leads the global market, driven by advanced digital health infrastructure, strong regulatory frameworks, and high clinical trial activity. The United States is the largest contributor, with widespread hospital adoption and deep integration into pharmaceutical research workflows.
Europe
Europe exhibits strong adoption driven by GDPR compliance requirements and expanding multinational clinical research programs. Countries such as Germany, the United Kingdom, and France are investing heavily in secure consent platforms aligned with data protection mandates.
Asia-Pacific
Asia-Pacific is the fastest-growing region, supported by expanding healthcare infrastructure, rising clinical trial outsourcing, and rapid mobile health adoption. China and India are emerging as high-potential markets due to large patient populations and government-led digital health initiatives.
Latin America and Middle East & Africa
These regions are witnessing gradual adoption, driven by regulatory modernization and growing interest in patient engagement technologies. Expansion is expected as digital health ecosystems mature and regulatory frameworks evolve.
Competitive Landscape
The eConsent market is highly competitive, featuring global health IT providers, clinical technology specialists, and emerging digital health startups. Key players differentiate through platform usability, regulatory expertise, integration capabilities, and global deployment support.
Strategic partnerships, product innovation, and acquisitions are central competitive strategies. Companies increasingly focus on integrated trial platforms, mobile-first solutions, and advanced analytics to strengthen customer value and retention.
Future Outlook
The EConsent in Healthcare Market is poised for sustained double-digit growth as digital transformation accelerates across healthcare delivery and clinical research. The convergence of telehealth, decentralized trials, patient engagement technologies, and regulatory digitization will continue to expand the role of electronic consent.
Future platforms will emphasize artificial intelligence–driven comprehension assessment, biometric identity verification, real-time protocol updates, and continuous consent management models. As healthcare systems prioritize transparency, efficiency, and patient empowerment, eConsent will evolve from a supporting tool into a core infrastructure component of digital healthcare.
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Spellen
- Gardening
- Health
- Home
- Literature
- Music
- Networking
- Other
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness
